Department Facilities

Translational Science Research Building that houses the department.

View of the East corridor from the North corner.

Common meeting-dinning area facing the corner of Union Avenue and S. Manassas Street.

View of the park from a typical room having six desks for fellows.

High-speed internet-connected desks for fellows (six cubicles per room).

Departmental cold room.

Departmental confocal microscope unit (Olympus FV1000 with 6 laser lines-405-458-488-514-559-635 nm). Please contact Dr. Tavalin for information.

Nanion Patchliner patch-clamp robot for high throughput screening of ion channels. Please contact Dr. Dopico or Dr. Bukiya for information.

Two computarized systems to measure in vitro myogenic tone. Please contact Dr. Bukiya or Dr. Dopico for information.

Two electrophysiology units for voltage- and current-clamp recordings. Please contact Dr. Tavalin for information.

Typical corridor joining the East and West sides of the Department. A high-speed internet connected desk on front left.

Typical patch-clamp station with computerized drug-delivery system. Please contact Dr. Dopico for information.

16 extra-tall Med-Associates operant chambers with two retractable levers and two sipper cups allowing concurrent operant self-administration of alcohol and alternative solutions. Eight chambers are outfitted with blue (473nm) lasers and eight are outfitted with green (532nm) lasers, allowing the delivery of laser stimulation under control of Med-Associates software for optogenetics experiments. Contact Dr. Brendan Tunstall for information.

32 standard Med-Associates operant chambers with two levers and two sipper cups allowing concurrent operant self-administration of alcohol and alternative solutions. Contact Dr. Brendan Tunstall for information.

Two alcohol-vapor exposure systems to allow passive exposure to cycles of alcohol vapor and regular air, under control of tablet computer. Each system is comprised of six airtight chambers, and able to chronically house 54 male or female rats at one time (i.e., 108 rats total). Contact Dr. Brendan Tunstall for information.

Surgical suite equipped with two rodent stereotaxic stations. Each station includes a dual-arm, digital-readout stereotaxic frame, equipped with dual micro-infusion pumps to allow bilateral micro-infusion (e.g., adeno-associated virus) or implantation (e.g., optic fiber) into brain regions of interest. Contact Dr. Brendan Tunstall for information.

Zebrafish Vivarium: The vivarium is equipped with two Semi-automated Tecniplast Aquatic Systems to house zebrafish in a controlled environment and a breeding and tank washing station.
Contact Dr. Vaithianathan for information.

Confocal microscope: Olympus FV-3000 RS confocal microscope-patch clamp electrophysiology unit to study high-resolution calcium and vesical dynamics in retinal neurons from zebrafish, mice, and rabbit animal models.
Contact Dr. Vaithianathan for information.

Multiphoton microscope: Olympus FVMPE-RS multiphoton-patch clamp electrophysiology unit to conduct in vivo and in vitro calcium signaling and dynamics of retinal synapses from zebrafish, mice, and rabbit models.
Contact Dr. Vaithianathan for information.
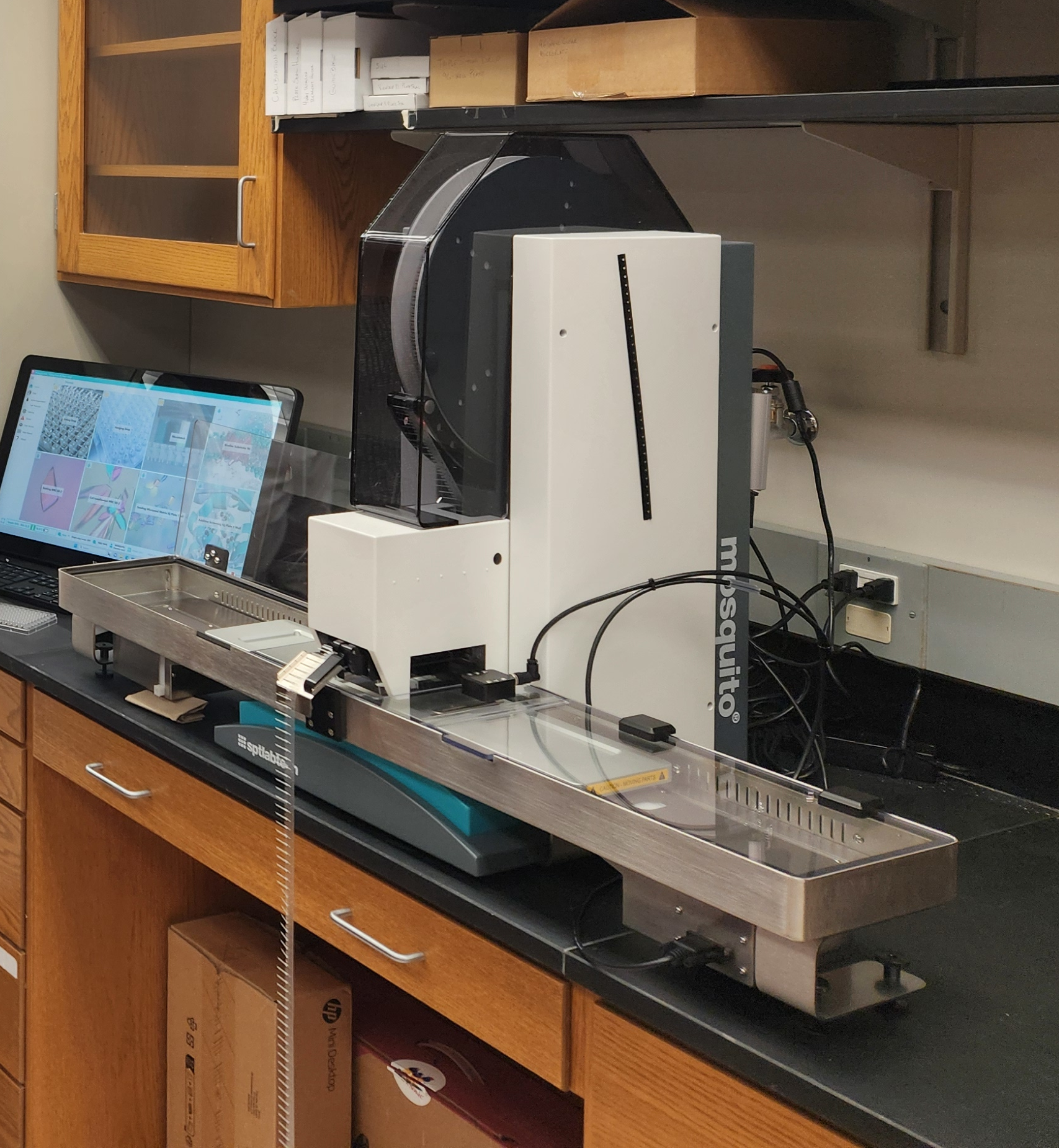
At the Structural Biology Core (SBC), which is at the center of cancer research, we
have mosquito Xtal3, an efficient crystallization robot. TheXtal3 provides accurate and precise multi-channel
pipetting from 500 nL to 5 µL, enabling researchers to benefit from miniaturization
in a variety of applications. The instrument's fast plate-to-plate pipetting motion
and tip interchange improve speed and efficiency. 1000's of screening crystallization
conditions can be set up in hours with great effectiveness.
Contact Dr. Jayaraman for information.