CITI Program Information
All UT Health Science Center College of Medicine and Erlanger campuses are committed to protecting our research volunteers and the integrity of the data derived from their participation in research. As a result, the university is requiring that its students, residents, fellows, faculty, investigators, IRB members, administrators, and all other individuals who participate in the conduct of human subject research, including those using human-derived materials, must complete a nationally recognized online course prior to beginning research at the university. The course is the Collaborative IRB Training Initiative (CITI) program. The CITI training program can be accessed here.
The UT Health Science Center College of Medicine-Chattanooga requires all incoming residents and fellows must complete the appropriate CITI modules before beginning training (prior to July 1).
There are a number of modules geared primarily to biomedical research and social/behavioral research. Review of all modules is encouraged, but learners are only required to complete the modules according to their category, which is defined by title/group (e.g. clinical investigators and staff, IRB members, IRB administrators, institutional officials or students), as indicated in the CITI introduction. BASIC Human Subject Research + Good Clinical Practice Modules are required.
Online training courses MUST be completed by:
- Anyone acting as an employee or agent of the UT Health Science Center College of Medicine-Chattanooga — including students, residents, fellows, and faculty/staff — engaged in the conduct of human subject research (working directly with human subjects or with identifiable data or biological specimens)
- Employees or agents conducting or assisting with human subjects research at any Erlanger campus, outpatient office or UT Health Science Center College of Medicine-Chattanooga facility.
- Department chairs who approve/acknowledge research
- Research administrators
- IRB board members and staff
If you have completed CITI at another institution, you can affiliate your certification with UT Health Science Center College of Medicine-Chattanooga by completing the following:
- Go to citiprogram.org/
- Log in with your username and password, which will take you to the main menu.
- In the blue banner, click “Add Institutional Affiliation”
- Select “University of Tennessee College of Medicine Chattanooga” from the list of institutions.
- Follow the online instructions
UT Health Science Center College of Medicine Chattanooga IRB approval of any new application, continuing review, or revision application is contingent upon training completion.
The University of Tennessee College of Medicine-Chattanooga Office of Human Subject Protections (OHSP) will administer this program. For questions or concerns regarding the CITI training program, please contact: Stacey Hendricks, 423.778.3818.
Instructions for CITI Registration
Log onto citiprogram.org
Once you access the CITI (Course in The Protection of Human Research Subjects) home page, you may follow the steps listed below to register the CITI Program.
Step 1: Click on the Register Icon to register yourself before you login.
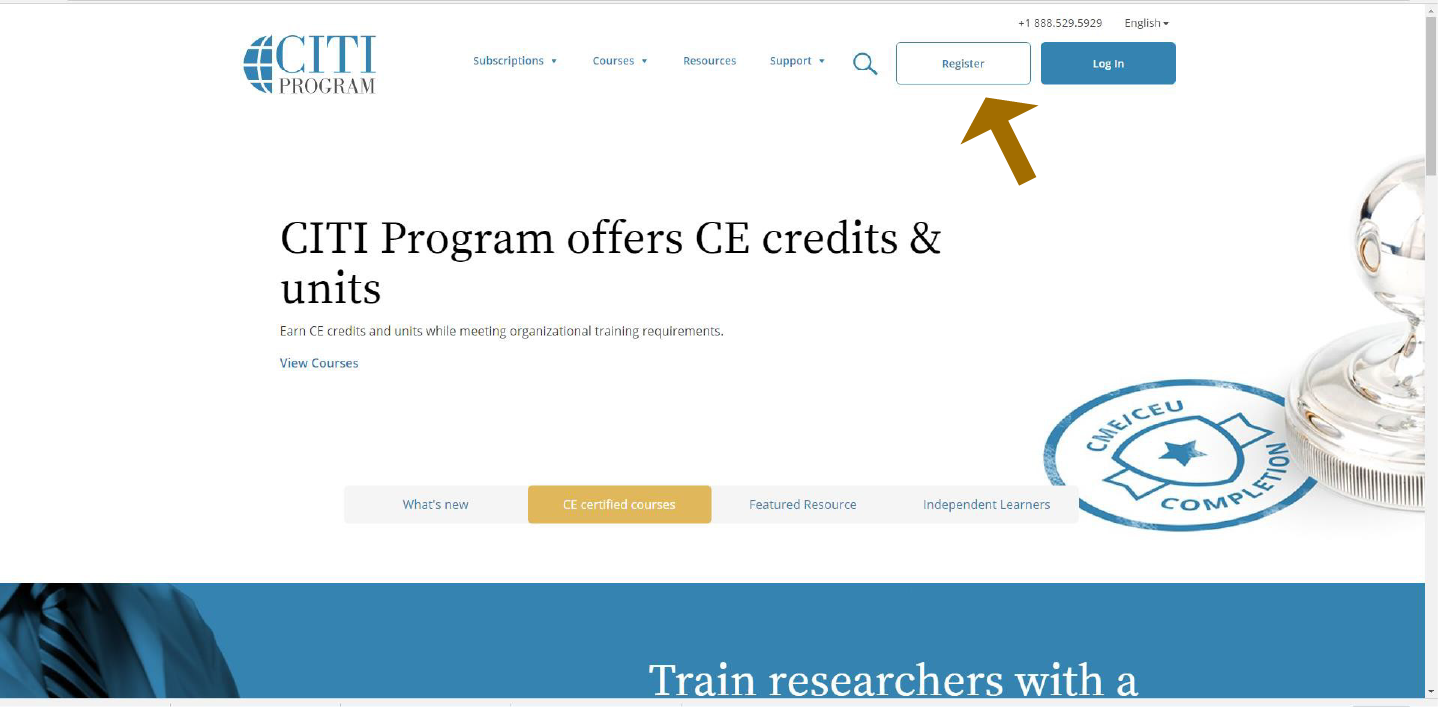
Step 2: To find our organization, start typing our name into the box provided (University of Tennessee College of Medicine Chattanooga) Don't get confused with UTC (local undergraduate campus). After you have entered the school name, agree to the terms and click on “Continue to Step 2”.

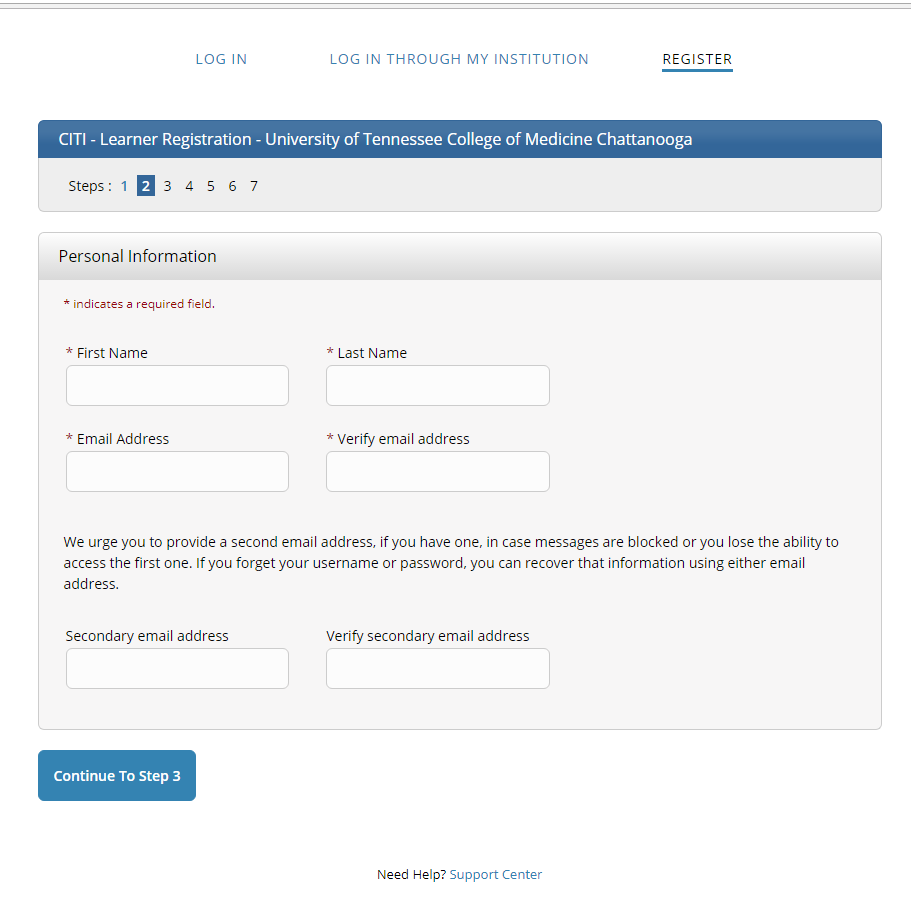
Step 3: Enter your name and email address (can be your personal email).
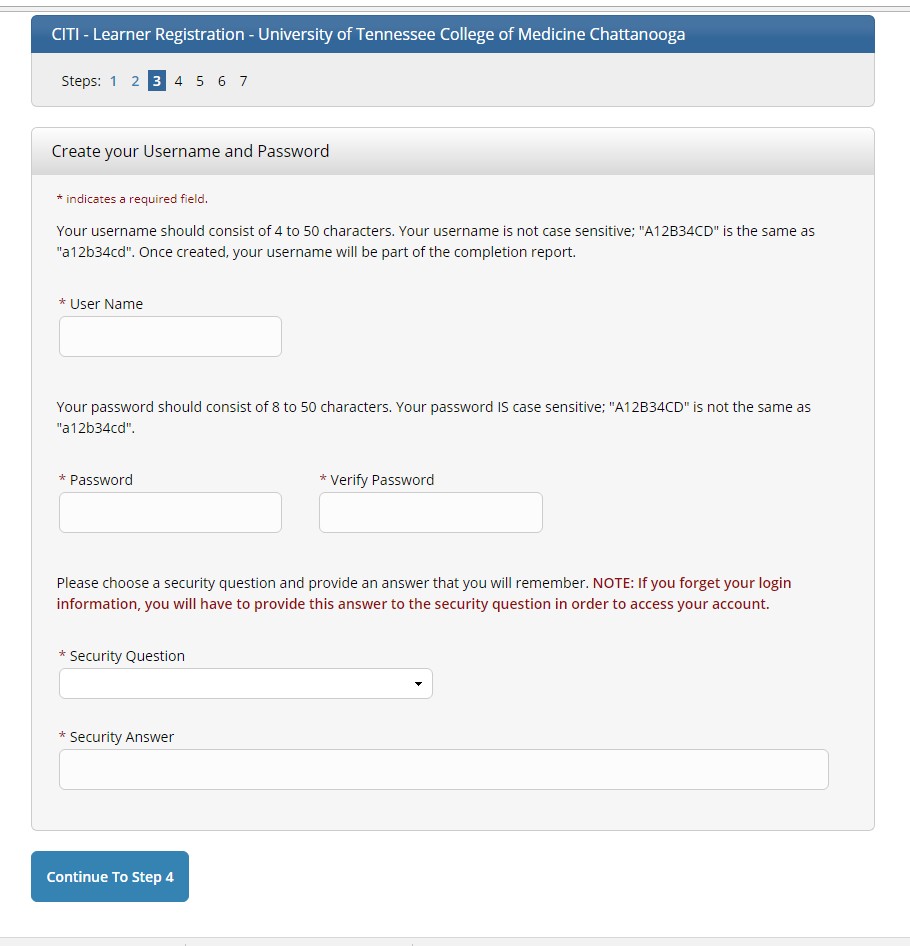
Step 4: Create a Username, Password, and set-up a Security Question.
Step 5: Enter your Country of Residence.

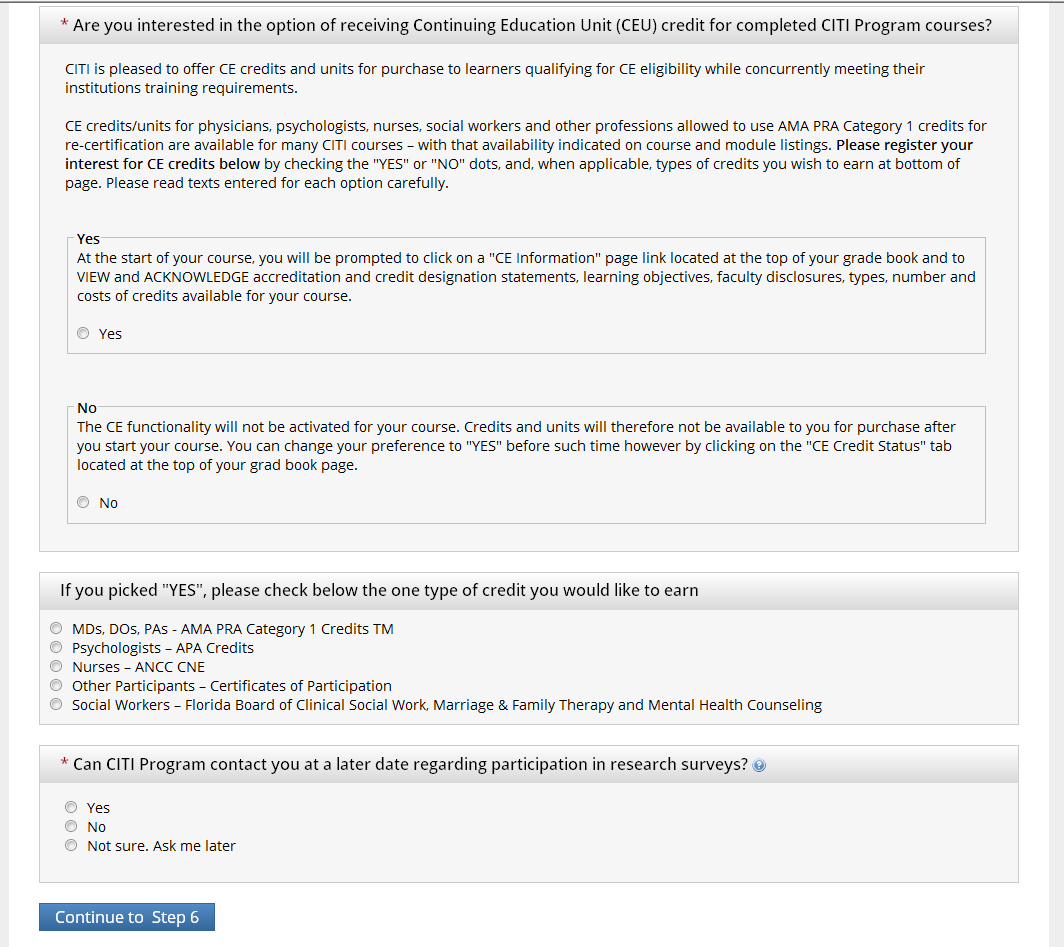
Step 6: Select “No” to receiving Continuing Education Unit credit for completed CITI Program Courses. Select if you would like to be contacted regarding research surveys.
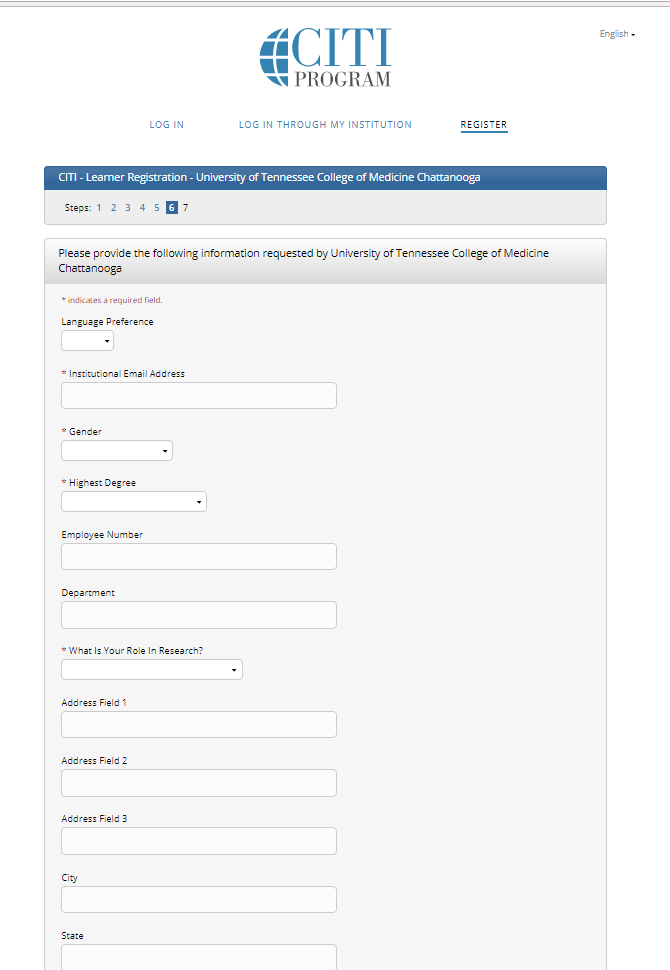
Step 7: Enter the required information requested by UT COM Chattanooga. For your role in research, select “Principal Investigator,” Co-Investigator,” or “Other” from the drop down list.
*You can use your personal email address for the institutional space (your Erlanger email address will not be active until you officially start).
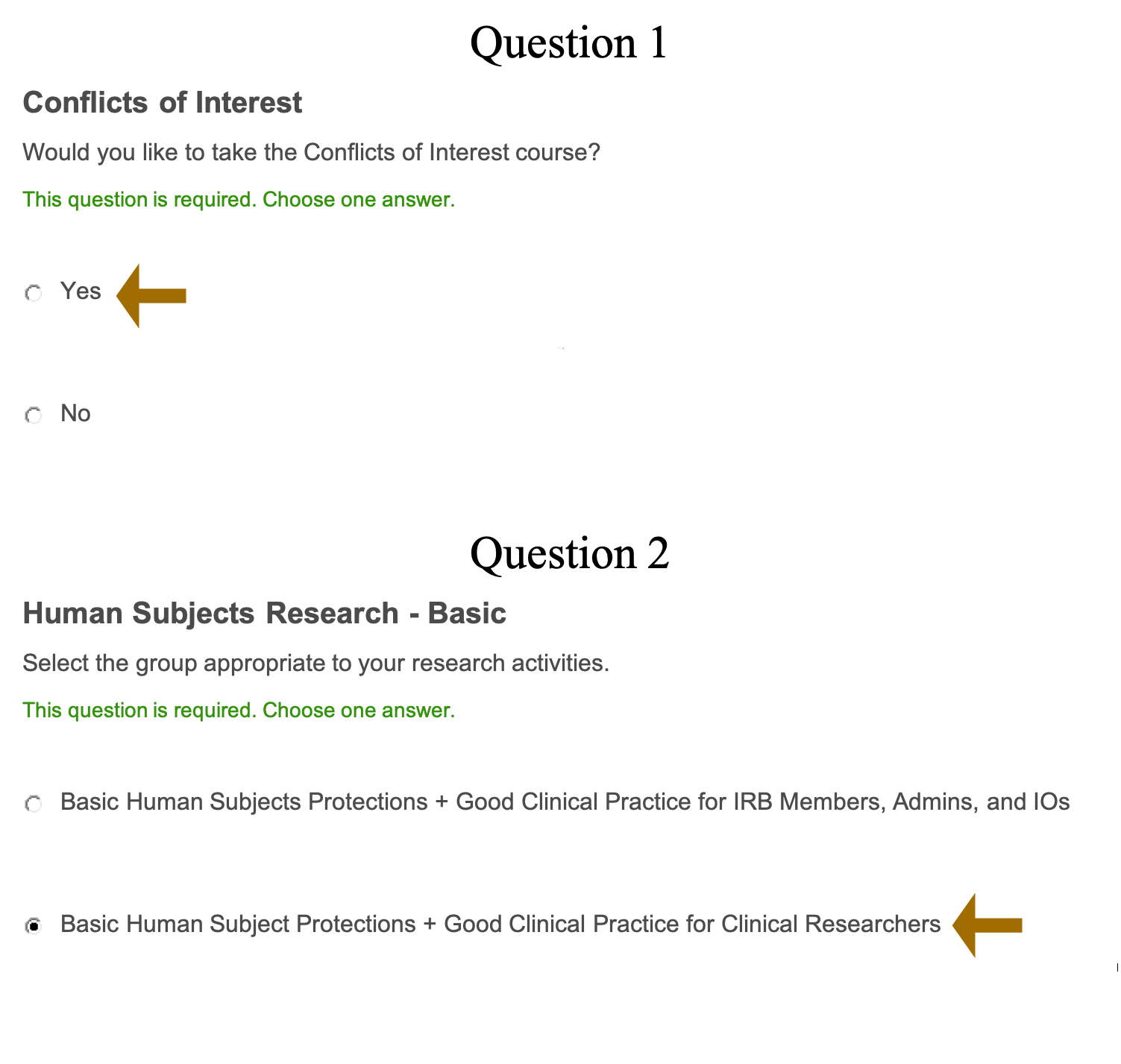
Step 8: Select “Yes” for the Conflicts of Interest course and Select Basic Human Subjects Research-Basic, as well as Good Clinical Practice for Clinical Researchers. After you select your required options, click complete registration.
Step 9: Click on “Finalize Registration.