Research Programs
Novel 3D Biofabrication Methodologies and Manufacturing for Enhanced Tissue Regeneration and Implantable Devices
Cell Interactions with 3D Bioprinted Vessels – Basic and Translational Approaches
This project’s goal is to study the cell interactions with 3D bioprinted blood vessel by using a system genetic tool, BXD mouse strains which is a unique genetic model that allows the identification of complex genetic traits regulating a biological response. For this purpose, the first step is to develop mouse model of vascular implantation using stem cell derived 3D bioprinted blood vessel. We have established the method of murine adipose tissue derived stem cell (mADSC) culture, propagation, and the assays to collect phenotypes such as regenerative potential of ADSCs. At the same time, we have developed the mouse model of blood vessel implantation by end-to-end anastomosis of infrarenal abdominal aorta of mice (Fig. 1). This truly unique and new mouse model provides us the chance of investigating the regulation of regeneration of bioprinted blood vessels in the BXD mouse strains. In collaboration with Revotek, the prototype of 3D bioprinter for small vessels has been developed and with a generous charitable grant shipped to Memphis in late June, 2020 (Fig. 2). This 3D bioprinter can print vessel with 1.2 mm outer diameter and 0.5 mm inner diameter as shown in Figure 3. This size closely resembles the size of a human coronary artery. Using the Biosynspherer just arrived to TennIRM, we now have the opportunity to develop customized bioink for use of mouse stem cells to create small blood vessels. We are now working on collecting ADSCs from parent strains of BXD (C57BL/6J and DBA/2J) and comparing the differences on cell surface markers, cell proliferation rate, and cell differentiation potential between strains and sexes. This work will help us to identify critical gene networks that regulate the regeneration of blood vessels. This information will provide novel insights into genetic manipulation and enhancement of 3D bioprinted blood vessels to further increase the success of implantation into human patients.
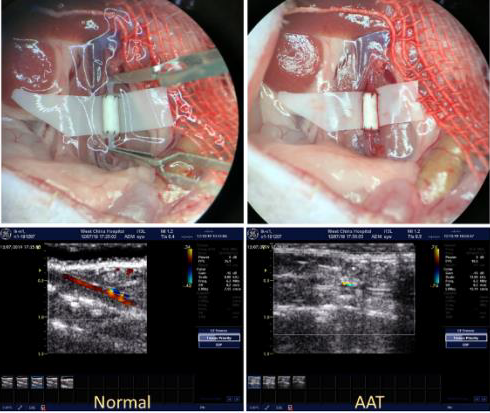 Figure 1. Mouse surgery of vessel graft implantation. Lower panel shows the blood
flow in the implanted graft by ultrasound.
Figure 1. Mouse surgery of vessel graft implantation. Lower panel shows the blood
flow in the implanted graft by ultrasound.
 Figure 2. Prototype of 3D bioprinter.
Figure 2. Prototype of 3D bioprinter.
 Figure 3. A 3D printed vessel with 1.2mm outer diameter, and 0.5mm inner diameter.
Figure 3. A 3D printed vessel with 1.2mm outer diameter, and 0.5mm inner diameter.
Stem Cell-Enhanced Tissue Regeneration: Engineering of Vascularized Bone/Cartilage Graft from Adipose-Derived Stem Cells
Harnessing Hematopoietic and Cancer-Stemlike Cells for Regenerative Medicine
The laboratory of Gabor Tigyi (UTHSC): Cancer stem-like cells (CSC) are a unique and small population of cells within a cancer that can self-renew, invade and also seed tumor metastasis in distant organs away from the primary tumor. The Tigyi group has been studying the mechanisms with which CSC develop resistance to chemo- and radiation-therapy. This research has identified the key role of the growth factor-like lipid mediator lysophosphatidic acid (LPA) in metastasis and therapy resistance. We have been pursuing a drug discovery research program aimed at developing inhibitors of the LPA type 2 receptor (LPAR2) which we identified plays a central role in ovarian and breast cancer metastasis and therapy resistance. We found that an LPAR2 inhibitor prototype compound designated as AM35 is very effective in killing CSC and enhance the effectiveness when co-applied with radiation and chemotherapy drugs. We also developed small molecule inhibitors of the autotaxin enzyme that generates LPA in carcinoma cells and in the cells of the tumor microenvironment. Two of our autotaxin inhibitors designated BMP-22 and BESA-3 have been found to be particularly effective in blocking the growth of CSC and metastasis in mouse carcinoma models. (Fig. 4).
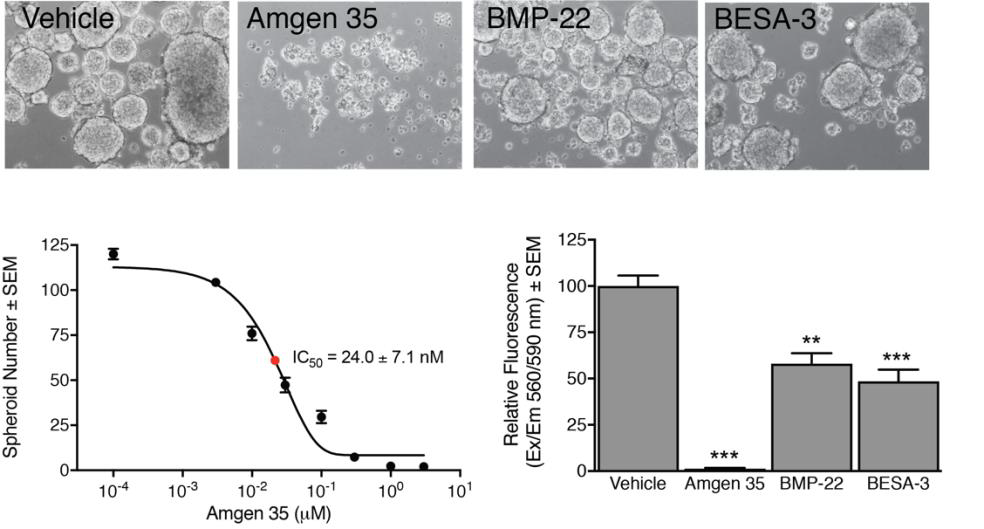 Figure 4. LPAR2 and autotaxin inhibition disrupts spheroid formation in 4T1 mouse
mammary carcinoma stem cells. CSCS form spheroids - mini tumors in culture - which
were disrupted by AM35, BMP-22 (ATX inhibitor) or BSEA-3 (mixed ATX/LPA1 inhibitor).
Cells were surveyed under 100X magnification (microscopy panels), and spheroids were
manually counted, plotted versus AM35 concentration with nanomolar potency (dose-response
panel). Cell viability was determined using Promega CellTiter Blue reagent as per
manufacturer's instructions. One-factor ANOVA with Bonferroni's post-test to determine
whether cell viability differed significantly between vehicle and drug treatment (**=p<0.01,
***=p<0.001; n=3).
Figure 4. LPAR2 and autotaxin inhibition disrupts spheroid formation in 4T1 mouse
mammary carcinoma stem cells. CSCS form spheroids - mini tumors in culture - which
were disrupted by AM35, BMP-22 (ATX inhibitor) or BSEA-3 (mixed ATX/LPA1 inhibitor).
Cells were surveyed under 100X magnification (microscopy panels), and spheroids were
manually counted, plotted versus AM35 concentration with nanomolar potency (dose-response
panel). Cell viability was determined using Promega CellTiter Blue reagent as per
manufacturer's instructions. One-factor ANOVA with Bonferroni's post-test to determine
whether cell viability differed significantly between vehicle and drug treatment (**=p<0.01,
***=p<0.001; n=3).
